T7 High Yield RNA Synthesis Kit—Efficient RNA Synthesis for Research
T7 High Yield RNA Synthesis Kit optimizes the transcription reaction system. The kit can synthesize the single-stranded RNA efficiently by using T7 RNA polymerase, the linear double-stranded DNA with the T7 promoter sequence as the template, and NTPs as the substrate to control the DNA sequence downstream of the promoter. During transcription, modified nucleotides can be added to the substrate to prepare biotin or dye-labeled RNA.
1. Introduction of T7 High Yield RNA Synthesis Kit
2. Principles of in vitro transcription
3. Advantages of Yeasen T7 High Yield RNA Synthesis Kit
4. Product performance
5. How to use this kit
6. Frequently Asked Questions
7. Ordering Information
1. Introduction of T7 High Yield RNA Synthesis Kit
T7 High Yield RNA Synthesis Kit can synthesize both long transcripts and short transcripts, and RNA can be produced 100-200 μg with 1 μg of DNA template input. The synthesized RNA can be used for various downstream applications, such as RNA structure and function research, RNase protection, probe hybridization, RNA interference, microinjection, and in vitro translation.
2. Principles of in vitro transcription
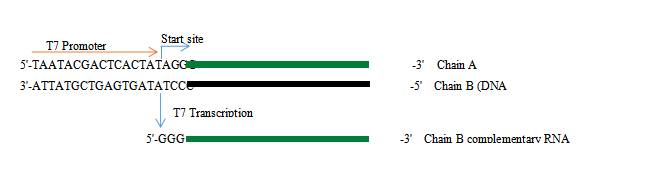
Figure 1. In vitro transcription process
3. Advantages of Yeasen T7 High Yield RNA Synthesis Kit
Extremely high yields—up to 200 µg in 2 hours
Versatile—suitable for 20nt-10000nt RNA transcripts
Multiple uses—generates unlabeled, labeled, or capped RNA
4. Product performance
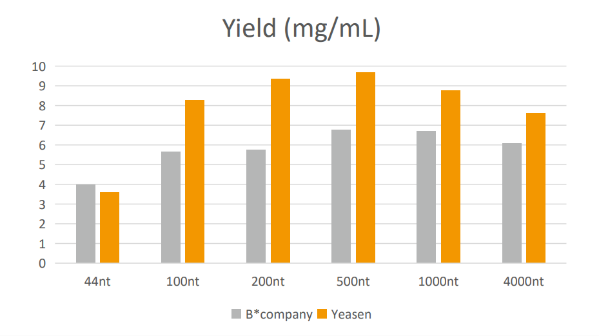
Figure 2. IVT yield comparison
Note: All reaction reagents are from T7 RNA Synthesis Kit (Yeasen#10623 or B* company). The IVT reaction yields of Yeasen and B* company are shown above.
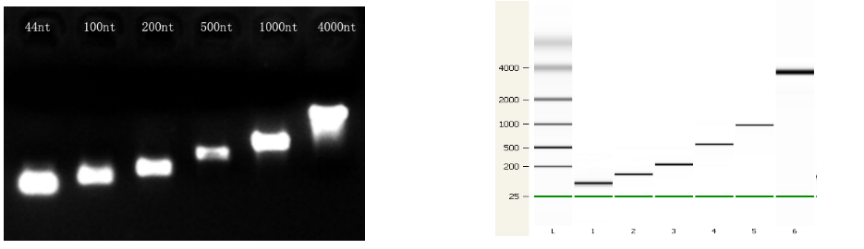
Figure 3. The quality of transcriptional products for different lengths
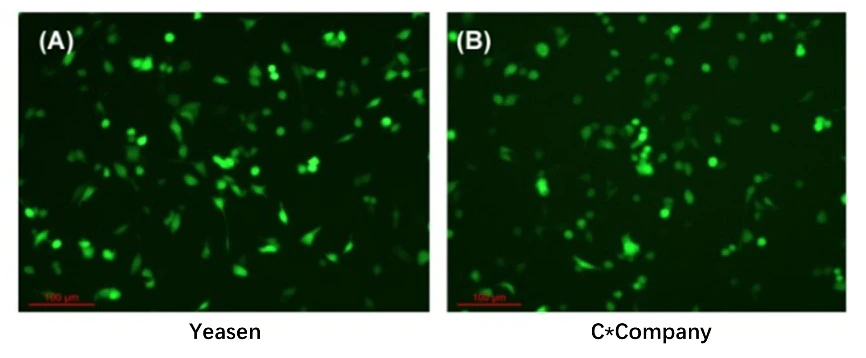
Figure 4. The expression level of the transcribed mRNA in HEK-293 cells
Note: The mRNA is Capped with Vaccinia Capping Enzyme (Yeasen#10614/10615)
5. How to use this kit
5.1 Thawing reagents
Centrifuge the T7 RNA Polymerase Mix briefly and place on ice. Thaw 10× Transcription Buffer and ribonucleotides (ATP, CTP, GTP, UTP), mix and centrifuge to the bottom of the tube, place 10× Transcription Buffer at room temperature and place four types of ribonucleotides on ice.
5.2 Prepare the transcription reaction system according to the following table
Table 1. Preparation method of the transcription reaction system
|
Components |
Volume(μL) |
Final concentration |
|
RNase free H2O |
Up to 20 |
- |
|
10×Transcription Buffer |
2 |
1× |
|
CTP / GTP/ ATP/ UTP (100 mM each) |
2 each |
10 mM each |
|
Template DNA |
1 µg |
- |
|
T7 RNA Polymerase Mix |
2 |
- |
Note:
a) The reaction is prepared at room temperature. Since the 10× Transcription Buffer contains spermidine, the concentration of spermidine too high at low temperatures will cause DNA template precipitation.
b) Short transcript (<100 nt), 2 µg template can be used, transcription time increased to 4-8 hs.
c) For long transcripts (>1000 nt), the linearized plasmid templates are recommended use for transcription.
d) Perform the reaction in the PCR machine with the hot lid open to prevent the reaction solution from evaporating for a long time.
e) The reaction product may have a white precipitate. This is magnesium pyrophosphate formed by free pyrophosphate and magnesium ions during the reaction, which won’t affect the subsequent experiments. You can add EDTA to remove it. If you are concerning the addition of EDTA affects subsequent experiments, the supernatant can also be recovered by centrifugation.
f) The reagents and containers should be without RNase contamination.
5.3 Incubate at 37°C for 2 hours
Mix the above reaction solution, briefly centrifuge to the bottom of the tube, and incubate at 37°C for 2 hs. If the transcript length is less than 100 nt, increase the reaction time to 4-8 hs.
5.4 DNase I treatment (optional)
After the reaction is completed, add 2 μL of DNase I (RNase free) to each tube and incubate at 37°C for 15 mins to remove the template DNA.
6. Frequently Asked Questions
6.1 The yield of the IVT reaction is low
The template quality is closely related to the yield. If the yield of the experimental group is significantly lower than that of the positive control group, the possible reasons can be.
① The templates contain components that will inhibit IVT reaction.
② There is something wrong with templates.
6.2 Suggestions
① Re-purify the template.
② Confirm the concentration and integrity of templates.
③ Extend the reaction time.
④ Increase the input of the template.
⑤Try other RNA polymerases and corresponding promoters.
6.3 The yield of short transcripts is low
If the target transcripts are shorter than 100 nt, extend the reaction time to 4-8 hours or increase the input of templates to 2ug in a 20 μL reaction system.
6.4 There are unexpected longer transcripts
If the result of gel electrophoresis shows that there are unexpected longer transcripts, the possible reasons can be:
① Plasmid templates might not be fully linearized.
②Templates have cohesive ends with 3’ overhangs.
③Transcripts have a secondary structure that is not completely denatured.
6.5 Suggestions
①Check whether plasmid templates are fully linearized. If necessary, perform plasmid linearization again.
②Select suitable restriction enzymes to linearize plasmid templates and avoid producing cohesive ends with 3’ overhangs. It is practicable to use Klenow fragment or T4 DNA polymerase to produce a blunt end if necessary.
③Use denatured gel to detect transcripts.
6.6 There are unexpected shorter transcripts
If the result of gel electrophoresis shows that there are unexpected shorter transcripts, the possible reasons can be:
①There is a sequence analogous to the termination sequence of T7 RNA polymerase in templates.
②The GC content of templates is high.
6.7 Suggestions
①Decrease the reaction temperature (such as 30℃) but note that sometimes decreasing the reaction temperature will reduce yields.
②Try other RNA polymerases to catalyze IVT reaction.
③If the GC content of templates is high, set the IVT reaction temperature at 42℃ or add SSB into the IVT reaction system to increase yields and the length of transcripts.
6.8 There is smearing of transcripts during gel electrophoresis
If there is the smearing of transcripts during gel electrophoresis, the possible reasons can be:
①There is RNase contamination during experimental operation.
②DNA templates are contaminated by RNases.
6.9 Suggestions
①Ensure all reagents are formulated with RNase-free H2O. Use RNase-free pipette tips and Eppendorf tubes and wear disposable latex gloves and masks during experimental operation.
②Re-purify DNA templates.
7. Ordering Information
The following are representative products offered by Yeasen. Additional sizes are available. Our products are highly optimized to work in concert, to help ensure superior performance and reproducibility.
We can also provide customized services. If you’re interested in a product that isn’t shown, contact us and we’ll work with you to meet your needs.
Table 2. Product information
Regarding reading:
GMP-grade reagents for mRNA in vitro synthesis
Yeasen Biotechnology GMP Grade mRNA In Vitro Synthesis Raw Materials
