Drug development requires animal models to test efficacy. With over 50 years of development and optimization, the Dextran Sulfate Sodium Salt (DSS) ulcerative colitis (UC) model, among various animal models, is widely used to study the etiology and pathogenesis of inflammatory bowel disease (IBD).
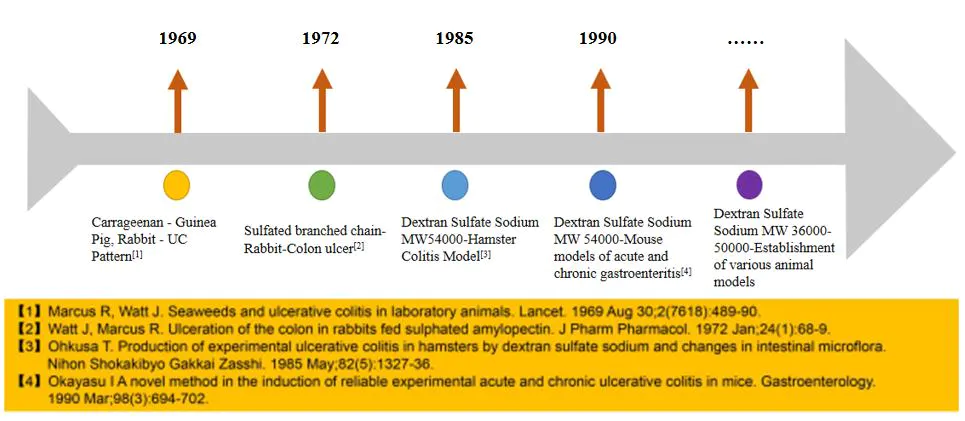
Figure 1. The development of the DSS ulcerative colitis model
1. Characteristics of the UC model constructed by DSS
2. Advantages of the DSS UC Model
3. DSS UC model construction example
4. Modelling success evaluation criteria
5. Links to this resource
6. Successful case for modeling with Yeasen DSS
7. FAQs
8. Product order
9. Published articles with our reagents
10. Regard Reading
1. Characteristics of the UC model constructed by DSS
Multiple acute or chronic symptoms of UC can be induced, such as diarrhea, mucus-like stool, fecal occult blood, gross bloody stool, weight loss, decreased activity, and poor coat color, by different DSS doses.
Table 1 Histological characteristics of the DSS colitis model
| DSS colitis model category | acute colitis model | Chronic phase colitis model |
|---|---|---|
| Histological changes | Colon hyperemia, edema, shortening, brittleness, increased weight-to-length ratio | Significantly shortened colon |
| Colon ulcers of varying degrees | Mucosal thickening, lymphadenopathy | |
| Mucosal edema, goblet cell loss, crypt swelling and destruction | Goblet cell loss, crypt loss | |
| Different degrees of inflammatory cell infiltration in the mucosa and submucosa, epithelial cell damage | Adenomatous polyps and tumor-like changes in a small number of animals |
2. Advantages of the DSS UC Model
👍 1. The protocols are easy to be implemented.
👍 2. The DSS UC model closely resembles human UC symptoms with high repeatability.
👍 3. Various characteristic symptoms can be induced by controlling the administrated DSS dose, which was unique for the DSS UC model.
👍 4. The DSS UC model can be generated with a variety of widely used model animals, such as mice, rats, zebrafish, pigs, fruit flies, etc.
👍 5. The IBD-induced colitis-associated cancer (CAC) model can be created with the combined use of azoxymethane (AOM).
3. DSS UC model construction example
3.1 Mouse model(Click for details)
1) BALB/c mice, female, 6-8 weeks, 25 g;
2) Sterile drinking water with 3% DSS, and filter with a 0.22 μm membrane;
3) DSS was administrated for 7 days;
4) Inflammation, such as colon edema and congestion, was observed after HE staining.
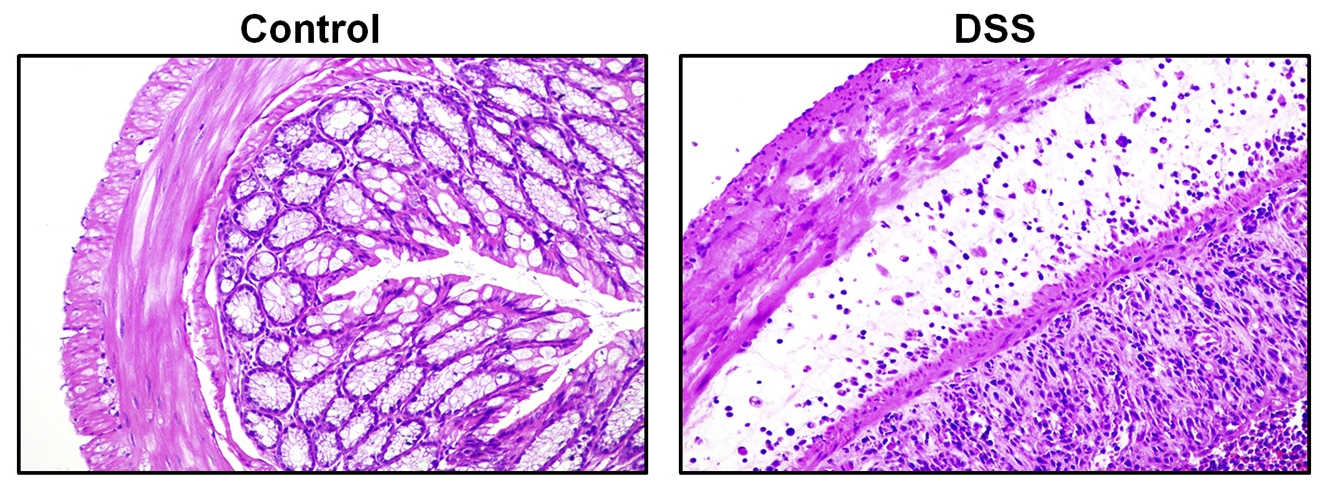
Fig.2 HE staining results of DSS acute colitis sections [1]
3.2 Zebrafish model(Click for details)
1) Zebrafish embryos were obtained from natural spawning and raised until 1 day post fertilization (dpf) in an incubator at 28.5℃ in recirculating water (60 μg/mL instant ocean sea salts) and supplemented with methylene blue up to 1 dpf;
2) After 1 dpf, use E3 embryo culture medium without methylene blue to culture to 3 dpf;
3) Prepare 10% DSS storage solution with E3 medium;
4) Dilute DSS with culture medium to the maximum non-lethal dose (DSS concentration reference: 0.5%);
5) The zebrafish were treated with 0.5% DSS from 3 dpf to 6 dpf, and the indexes were observed.
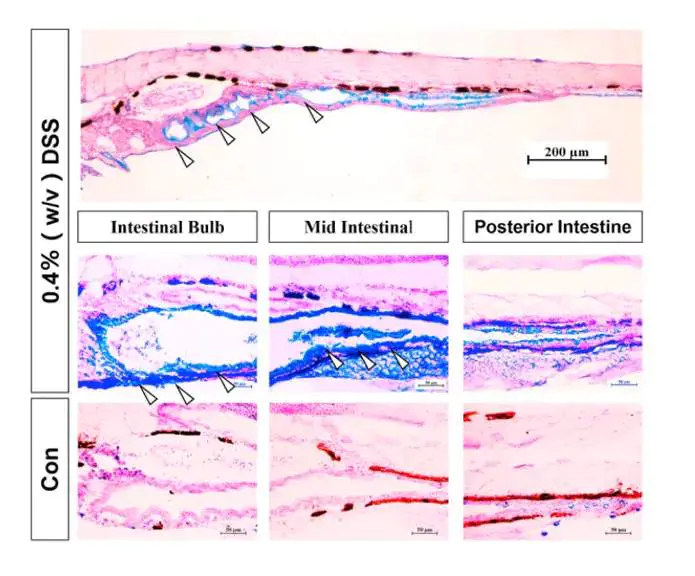
Fig.3 DSS induces an inflammatory response in zebrafish liver [2]
3.3 Pig model(Click for details)
1) Yorkshire piglets at age of 4-5 days;
2) DSS dose: 1.25g/kg, oral intake for 5 days;
3) Increased D-mannitol uptake rate was observed.
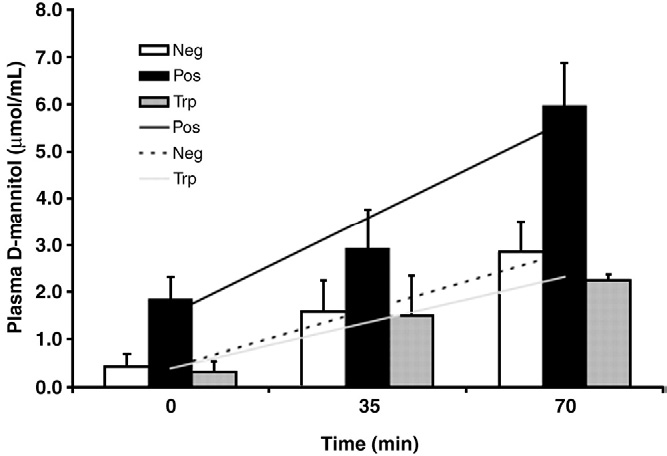
Fig.4 DSS-induced D-mannitol concentration in piglets was higher than that in the control group [3]
3.4 Drosophila model(Click for details)
1) Drosophila, female, 5-10 days;
2) Feeding medium was prepared with 5% sucrose solution containing 3% DSS and 25 μg/mL bleomycin;
3) Drosophila was cultured at 29°C for three days with a daily change of culture bottle;
4) DSS induced the proliferation of ISC precursor cells and had a lethal effect on drosophila.
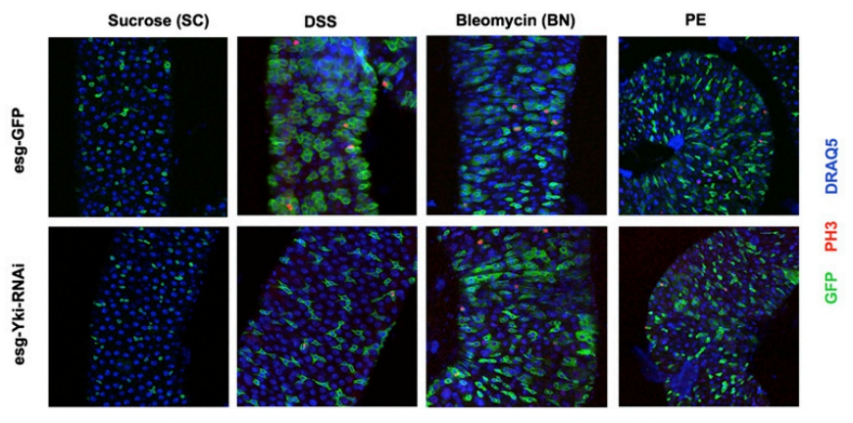
Fig.5 DSS induces the proliferation of ISC precursor cells in Drosophila [4].
3.5 Colitis associated cancer(Click for details)
1)BALB/c mice, male,7 weeks old;
2)On day 1, weigh and mark mice. Inject mice intraperitoneally with 10 mg/kg AOM solution.
3) Fill the drinking supply of the mouse cages with water. Calculate 7-10 mL water per mouse per day. Feeding for one week.
4) Replace drinking water with 2.5% DSS for one week;
5) Feed with regular drinking water for two weeks;
6) Repeat steps 3-4 for 3 times.
![Fig.6 Schematic diagram of CAC induced by AOM/DSS [5] .](https://seas.ysbuy.com/file/32626/24d9-166605804688444368.jpg?imageView2/2/format/webp/q/100)
Fig.6 Schematic diagram of CAC induced by AOM/DSS [5] .
4. Modelling success evaluation criteria
4.1 Disease Activity Index (DAI score)
The model was evaluated with multiple parameters including body weight, fecal viscosity, and occult blood, through which generates the overall DAI.
Table 2 DAI scoring rules
|
score |
percent weight loss |
stool consistency |
fecal occult blood |
|---|---|---|---|
|
0 |
0 |
Normal |
Negative |
|
1 |
1-5% |
Soft stool |
Light blue |
|
2 |
5-10% |
Mucoid stool |
Blue |
|
3 |
10-20% |
Loose stools |
Dark blue |
|
4 |
>20% |
\ |
Gross bloody stool |
4.2 Histological Change Score
The scores were given for the above indicators based on the histological changes excluding the lymph node formation for the acute colitis model. The HE staining reagent used is Cat#60524ES60.
Table 3 Histological change score
|
Sore Ulcer |
Ulcer ( s )
|
Epichanges inflammatory |
Inflammatory infiltrate |
Lymph node( s )
|
|---|---|---|---|---|
|
0 |
0 |
Normal |
None |
none |
|
1 |
1 |
Goblet cell loss |
Pericrypt infiltration |
1 |
|
2 |
2 |
Goblet cell loss |
Infiltration of the muscular mucosae |
2 |
|
3 |
3 |
Crypt deletion |
General infiltration of the muscularis mucosa, thickening of the mucosa |
3 |
|
4 |
>3 |
Extensive crypt loss or polypoid regeneration |
Submucosa invasion |
> |
4.3 Colon length
The shortened colon length was an obvious phenotype for the chronic colitis model, while similar changes can be detected on day 8 for the acute colitis model.
4.4 Summary
Preliminary experiments are recommended for using the DSS UC animal model with appropriate control. 8-10 animals per group are minimal requirements. DAI is the criteria to evaluate the results from a preliminary study.
5. Links to this resource
[1]Xiaona Gao, et al. Journal of Nutritional Biochemistry 83 (2020) 108438.
[2]Jing Ma, et al. Aquaculture and Fisheries (2021) 548–557.
[3]Connie J. Kim, et al. Journal of Nutritional Biochemistry 21 (2010) 468–475.
[4]Fangfang Ren,et al.PNAS.2010.107 (49) 21064-21069.
[5]Jia-Rong Huang, et al.Frontiers in Pharmacology.2020.11:586885.
6. Successful case for modeling with Yeasen DSS
Successful acute colitis models are generated with Yeasen DSS (Cat#60316ES, MW: 36000~50000) in 7 days with prominent phenotype as shown in the following table.
Table 4 Construction of different types of enteritis models with DSS
|
Model |
Modeling samples |
Modeling plan |
Modeling results |
Use evaluation |
|---|---|---|---|---|
|
Acute colitis |
BALB/c mice, female, 6-8 weeks, 25 g |
3%-5% DSS drink freely for 7 consecutive days |
Day 5 appeared, the length of the colon was shortened, HE staining, and the inflammation was obvious |
Molding speed is fast and the time is short. Consistent with the characteristics of acute colitis model |
|
C57BL/6 mice, male, 8 weeks, 20 g |
3%-5% DSS by gavage, continuous administration |
Day 5 occurs, colon shortening, weight loss, blood in the stool, diarrhea |
High mold rate, and short duration. Consistent with the characteristics of acute colitis model |
|
|
Chronic colitis |
C57BL/6 mouse, male, 8 weeks, 22 g |
1-2% DSS by gavage, continuous administration |
Day40 appears, colon shortening, weight loss, blood in the stool, diarrhea |
High molding rate . Consistent with the characteristics of the chronic colitis model |
|
Colon cancer |
C57BL/6 mouse, male, 8 weeks, 21 g |
1%-2% DSS ad libitum for 5 days for 3 weeks |
14 weeks with shortened colon length, weight loss, HE staining, and obvious inflammation |
High molding rate . Consistent with colon cancer model characteristics |
7. FAQs
Regardless of the acute DSS colitis model or chronic DSS colitis model, the severity and success of enteritis are related to mouse species (different genetic backgrounds), DSS concentration, and dosing cycle.
Table 5 Common problems of DSS colitis modeling
|
Possible problems |
Possible reason |
Suggested solution |
|---|---|---|
|
High mortality in mice |
DSS concentration too high |
Decrease the concentration of DSS administered |
|
Mice with no or low symptoms of enteritis |
DSS concentration too low |
Increase DSS dosing concentration; decrease cycle interval (10-14 days) |
|
In the same group of mice, the symptoms of enteritis vary greatly |
Bottle cap clogged |
Check the mouse drinking bottle daily |
8. Product order:
Hot-selling product only needs 1/3 of the price of M* with the same efficiency, and we keep a large stock.
Table 6 Product Order
| Product Name | Cat NO. | Size |
|
ColitCareTM Dextran Sulfate Sodium Salt (DSS), Colitis Grade MW:36000~50000 |
60316ES25 | 25g |
| 60316ES60 | 100 g | |
| 60316ES76 | 500 g | |
| 60316ES80 | 1 kg |
9. Published articles with our reagents:
2024
[1]Zhong D, Jin K, Wang R, ,et al. Microalgae-Based Hydrogel for Inflammatory Bowel Disease and Its Associated Anxiety and Depression. Adv Mater. 2024 Jan 26:e2312275. doi: 10.1002/adma.202312275. IF=29.4
[2] Zhang Y, Tu S, Ji X, Wu J, Meng J, Gao J, Shao X, Shi S, Wang G, Qiu J, Zhang Z, Hua C, Zhang Z, Chen S, Zhang L, Zhu SJ. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat Commun. 2024 Feb 13;15(1):1333. doi: 10.1038/s41467-024-45636-x. IF= 16.6
2022
[1]Mengmeng Xu, Ying Kong, Nannan Chen,et al.Identification of Immune-Related Gene Signature and Prediction of CeRNA Network in Active Ulcerative Colitis[J].Frontiers in Immunology.2022; 13: 855645. IF=7.561
[2]Lujuan Xing, Lijuan Fu, Songmin Cao,et al.The Anti-Inflammatory Effect of Bovine Bone-Gelatin-Derived Peptides in LPS-Induced RAW264.7 Macrophages Cells and Dextran Sulfate Sodium-Induced C57BL/6 Mice[J]. Nutrients 2022, 14, 1479. IF=5.717
[3]Wang S, Huang J, Tan KS, et al.Isosteviol Sodium Ameliorates Dextran Sodium Sulfate-Induced Chronic Colitis through the Regulation of Metabolic Profiling, Macrophage Polarization, and NF-B Pathway[J].Oxidative Medicine and Cellular Longevity. 2022,4636618. IF=5.076
[4]YuangengLi, PingYu, WenwenFu, et al.Polysaccharides from Panax ginseng C. A. Meyer alleviated DSS-induced IBD by inhibiting JAK2/STAT1/NLPR3 inflammasome signalling pathway in mice[J].Journal of Functional Foods.2022, 105013. IF=4.451
[5]Lei-NingChen, TaoJing, Zi-BinLin, et al.Metabolomic and transcriptomic responses of mouse testis to the dextran sulfate sodium induced colitis[J].Reproductive Toxicology.2022, Pages 35-42. IF=3.143
2021
[1]Li Zhao, Fei Wang, Zhengwei Cai, et al.Improving drug utilization platform with injectable mucoadhesive hydrogel for treating ulcerative colitis[J]. chemical engineering journal.424(2021)130464.IF=16.744
[2]Lingjun Tong, Haining Hao, Zhe Zhang, et al.Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota[J].Theranostics.2021; 11(17): 8570-8586 IF=11.556
[3]Jingjing Gan, Yuxiao Liu, Lingyu Sun, et al.Orally administrated nucleotide-delivery particles from microfluidics for inflammatory bowel disease treatment[J]. Applied Materials Today.2021 Dec;25:101231. IF=10.041
[4]JialiDong, YuanLi,HuiwenXiao, et al.Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models[J].Cell reports.2021, 109886.IF=9.423
[5]Hao H, Zhang X, Tong L, Liu Q, et al.Lactobacillus plantarumEffect of Extracellular Vesicles Derived From Q7 on Gut Microbiota and Ulcerative Colitis in Mice[J].Frontiers in Immunology.2021.777147. IF=7.561
[6]Yaohua Fan, Yanqun Fan, Kunfeng Liu, et al.Edible Bird’s Nest Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice by Restoring the Th17/Treg Cell Balance[J].Frontiers in Pharmacology.2021.632602. IF=7.561
2020
[1]Li, Y., Dong, J., Xiao, H., Zhang, S., Wang, B., Cui, M., & Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes,.2020 .1–18.IF=10.245
[2]Jia-Rong Huang, Sheng-Te Wang, Meng-Ning Wei, et al.Piperlongumine Alleviates Mouse Colitis and Colitis-Associated Colorectal Cancer[J].Frontiers in Pharmacology.2020.586885. IF=7.561
[3]Gao X, Fan W, Tan L, et al. Soy isoflavones ameliorate experimental colitis by targeting ERα/NLRP3 inflammasome pathways[J]. The Journal of Nutritional Biochemistry, 2020, 83. IF=6.048
Before 2020
[1] Oehlers SH, Flores MV, Hall CJ, Crosier KE, Crosier PS. Retinoic acid suppresses intestinal mucus production and exacerbates experimental enterocolitis. Dis Model Mech. 2012 Jul;5(4):457-67. IF=4.973
[2] Kim CJ, Kovacs-Nolan JA, Yang C, Archbold T, Fan MZ, Mine Y. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem . 2010 Jun;21(6):468-75. IF=6.048
[3] Karpowicz , P., Perez, J. & Perrimon , N.,. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development (Cambridge, England), 2010,137(24), pp.4135–4145. IF=6.868
[4] Fan H, Chen W, Zhu J, et al. Toosendanin alleviates dextran sulfate sodium-induced colitis by inhibiting M1 macrophage polarization and regulating NLRP3 inflflammasome and Nrf2/HO-1 signaling[J]. International immunopharmacology, 2019, 76: 105909. IF=3.943
Regard Reading
The protocol of Ulcerative Colitis Zebrafish Modeling using Dextran sodium sulfate (DSS)
The protocol of Ulcerative Colitis Drosophila Modeling using Dextran sodium sulfate (DSS)
The protocol of Ulcerative Colitis Piglet Modeling using Dextran sodium sulfate (DSS)
