Increased intestinal permeability is one of the etiological factors of IBD and, thus, can be used as a biomarker to assess the severity of small and large intestinal damage. D-Mannitol, an inert small carbohydrate molecule that can be absorbed along the entire crypt villus axis, was used to assess in vivo intestinal permeability[1].

Figure 1. Piglet
1. Model building
2. Results
3. Links to this resource
4. Successful case for modeling with Yeasen DSS
5. Product order
6. Published articles with our reagents
7. Regard Reading
1. Model building
1.1 Animal Model
Yorkshire piglets at age of 4-5 days
1.2 Material
DSS(Yeasen#60316ES,MW36000-50000)
1.3 Protocol
a) Yorkshire piglets were housed individually in metal floor pens with rubberized floors in a controlled 12 h light/dark cycle room. Room temperature was maintained at 26℃ and supplemented with the heating lamp;
b) Piglets were fed a commercial milk replacer formula diet three times a day, similar to their ad libitum intake level;
c) Animals were surgically fitted with an intragastric catheter, The catheter was anchored to a silicone patch (about 8×12 mm), which was further sutured onto the gastric wall with approximately 30 mm inserted inside the gastric lumen. Each animal was fitted in a custom-made vest with a dorsal pocket for the temporary storage of the exterior segment of the catheter;
d) Animals were divided into a positive control group (Pos), negative control group (Neg), and experimental group (Trp);
e) Positive control group (Pos): perfusion DSS(5 days)+saline(5 days); Negative control group (Neg): perfused with normal saline(10 days); Experimental group (Trp): infuse DSS(5 days)+therapeutic drugs(5 days);
f) DSS dose: 1.25g/kg, oral intake for 5 days;
g) At the endpoint, animals were sedated through an inhaled anesthetic, isoflurane, and euthanized via intracardiac injection of Ethanol (pentobarbital) at 0.3 ml/kg·body weight (BW).
h) Colon tissues were immediately rinsed in cold saline solution (154 mM, pH7.4) containing 0.1 mM phenylmethyl sulfonyl fluoride (PMSF) and sampled for histology or flash frozen in liquid nitrogen for subsequent analysis.
2. Results
2.1 Intestinal permeability analysis
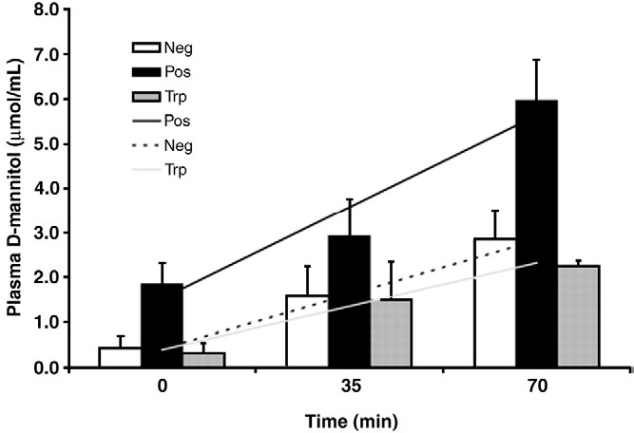
Figure 2. DSS-induced D-mannitol concentration in piglets was higher than that in the control group
DSS-induced D-mannitol concentration in piglets increased and the intestinal barrier was destroyed.
2.2 H&E pathological staining section
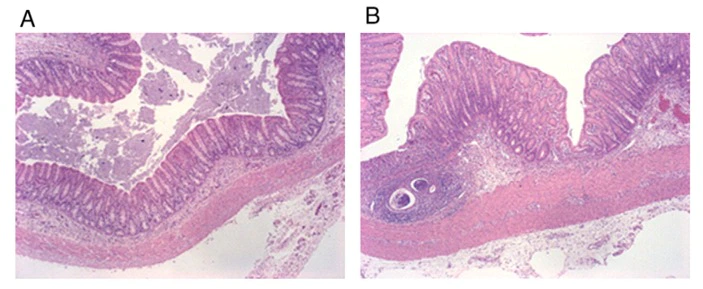
Figure 3. H&E pathological staining section (A: Neg group; B: Pos group)
The severity of colonic inflammation was assessed by inflammation scoring and histological measurements. In the Pos group treated with DSS, the recess structure was distorted, inflammatory cells infiltrated into mucosa and submucosa, recess abscess, and recess inflammation.
2.3 Detection of inflammatory factors
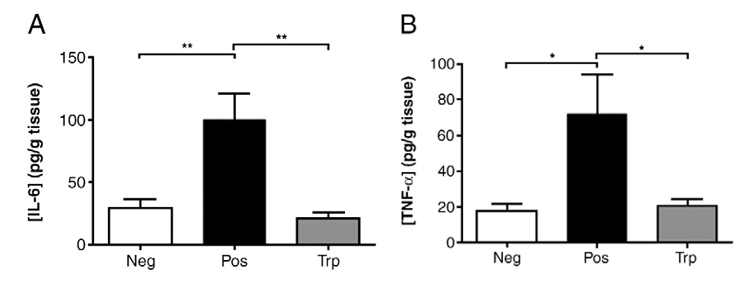
Figure 4. Detection of inflammatory factors
The concentration of two cytokines in the Pos group treated with DSS increased[1].
3. Links to this resource
[1]Connie J. Kim, et al. Journal of Nutritional Biochemistry 21 (2010) 468–475.
4. Successful case for modeling with Yeasen DSS
Yeasen provides high-quality DSS (Cat#60316ES): purity (98%), sulfur content 17-19%, free sulfur <0.2%, supported by a large number of literature data.
Table 1 Construction of different types of enteritis models with DSS
|
Model |
Modeling samples |
Modeling plan |
Modeling results |
Use evaluation |
|
acute colitis |
BALB/c mice, female, 6-8 weeks, 25 g |
3%-5% DSS drink freely for 7 consecutive days |
On day 5 appeared the length of the colon was shortened, HE staining, and the inflammation was obvious |
molding speed is fast and the time is short. Consistent with the characteristics of the acute colitis model |
|
C57BL/6 mice, male, 8 weeks, 20 g |
3%-5% DSS by gavage, continuous administration |
Day 5 occurs, colon shortening, weight loss, blood in the stool, diarrhea |
High mold rate, and short duration. Consistent with the characteristics of the acute colitis model |
|
|
chronic colitis |
C57BL/6 mouse, male, 8 weeks, 22 g |
1-2% DSS by gavage, continuous administration |
Day40 appears, colon shortening, weight loss, blood in the stool, diarrhea |
High molding rate. Consistent with the characteristics of the chronic colitis model |
|
colon cancer |
C57BL/6 mouse, male, 8 weeks, 21 g |
1%-2% DSS ad libitum for 5 days for 3 weeks |
14 weeks with shortened colon length, weight loss, HE staining, and obvious inflammation |
High molding rate. Consistent with colon cancer model characteristics |
5. Product order
Hot-selling product only needs 1/3 of the price of M* with the same efficiency, and we keep a large stock.
Table 2. Product Order
| Product Name | Cat NO. | Size |
|
ColitCare™ Dextran Sulfate Sodium Salt (DSS), Colitis Grade MW:36000~50000 |
60316ES25 | 25g |
| 60316ES60 | 100 g | |
| 60316ES76 | 500 g | |
| 60316ES80 | 1 kg |
6. Published articles with our reagents
[1]Li Zhao, Fei Wang, Zhengwei Cai, et al.Improving drug utilization platform with injectable mucoadhesive hydrogel for treating ulcerative colitis[J]. chemical engineering journal.424(2021)130464.IF=16.744
[2]Lingjun Tong, Haining Hao, Zhe Zhang, et al.Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota[J].Theranostics.2021; 11(17): 8570-8586 IF=11.556
[3]Li, Y., Dong, J., Xiao, H., Zhang, S., Wang, B., Cui, M., & Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes,.2020 .1–18.IF=10.245
[4]Jingjing Gan, Yuxiao Liu, Lingyu Sun, et al.Orally administrated nucleotide-delivery particles from microfluidics for inflammatory bowel disease treatment[J]. Applied Materials Today.2021 Dec;25:101231 IF=10.041
[5]Mengmeng Xu, Ying Kong, Nannan Chen,et al.Identification of Immune-Related Gene Signature and Prediction of CeRNA Network in Active Ulcerative Colitis[J].Frontiers in Immunology.2022; 13: 855645. IF=7.561
[6]JialiDong, YuanLi, HuiwenXiao, et al.Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models[J].Cell reports.2021, 109886.IF=9.423
[7]Hao H, Zhang X, Tong L, Liu Q, et al.Lactobacillus plantarumEffect of Extracellular Vesicles Derived From Q7 on Gut Microbiota and Ulcerative Colitis in Mice[J].Frontiers in Immunology.2021.777147 .IF=7.561
[8]Yaohua Fan, Yanqun Fan, Kunfeng Liu, et al.Edible Bird’s Nest Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice by Restoring the Th17/Treg Cell Balance[J].Frontiers in Pharmacology.2021.632602.IF=7.561
[9]Jia-Rong Huang, Sheng-Te Wang, Meng-Ning Wei, et al.Piperlongumine Alleviates Mouse Colitis and Colitis-Associated Colorectal Cancer[J].Frontiers in Pharmacology.2020.586885. IF=7.561
[10]Gao X, Fan W, Tan L, et al. Soy isoflavones ameliorate experimental colitis by targeting ERα/NLRP3 inflammasome pathways[J]. The Journal of Nutritional Biochemistry, 2020, 83.IF=6.048
[11]Lujuan Xing, Lijuan Fu, Songmin Cao,et al.The Anti-Inflammatory Effect of Bovine Bone-Gelatin-Derived Peptides in LPS-Induced RAW264.7 Macrophages Cells and Dextran Sulfate Sodium-Induced C57BL/6 Mice[J]. Nutrients 2022, 14, 1479. IF=5.717
[12] Wang S, Huang J, Tan KS, et al.Isosteviol Sodium Ameliorates Dextran Sodium Sulfate-Induced Chronic Colitis through the Regulation of Metabolic Profiling, Macrophage Polarization, and NF-B Pathway[J].Oxidative Medicine and Cellular Longevity. 2022,4636618. IF=5.076
7. Regard Reading
Establishment of Dextran sodium sulfate (DSS) Ulcerative Colitis Model
Establishment of Azoxymethane (AOM) and Dextran Sulfate Sodium (DSS) Induced Colitis-Associated Cancer Model
The protocol of Ulcerative Colitis Drosophila Modeling using Dextran sodium sulfate (DSS)
The protocol of Ulcerative Colitis Zebrafish Modeling using Dextran sodium sulfate (DSS)
