HCD/HCP

High-sensitivity, fg-grade, residual DNA detection kits for quality control of biological products
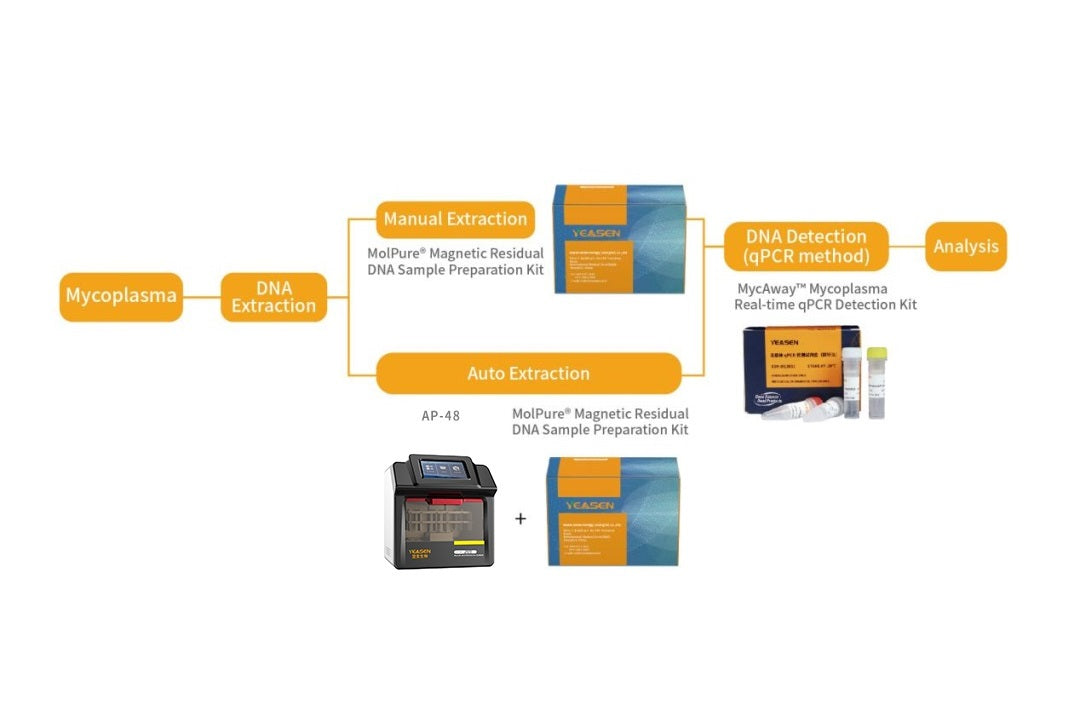
MycAway Mycoplasma Real-time qPCR Detection Kit (2G) Validation Report
CHO Host Cell DNA Residue Detection Kit (3G) Validation Report
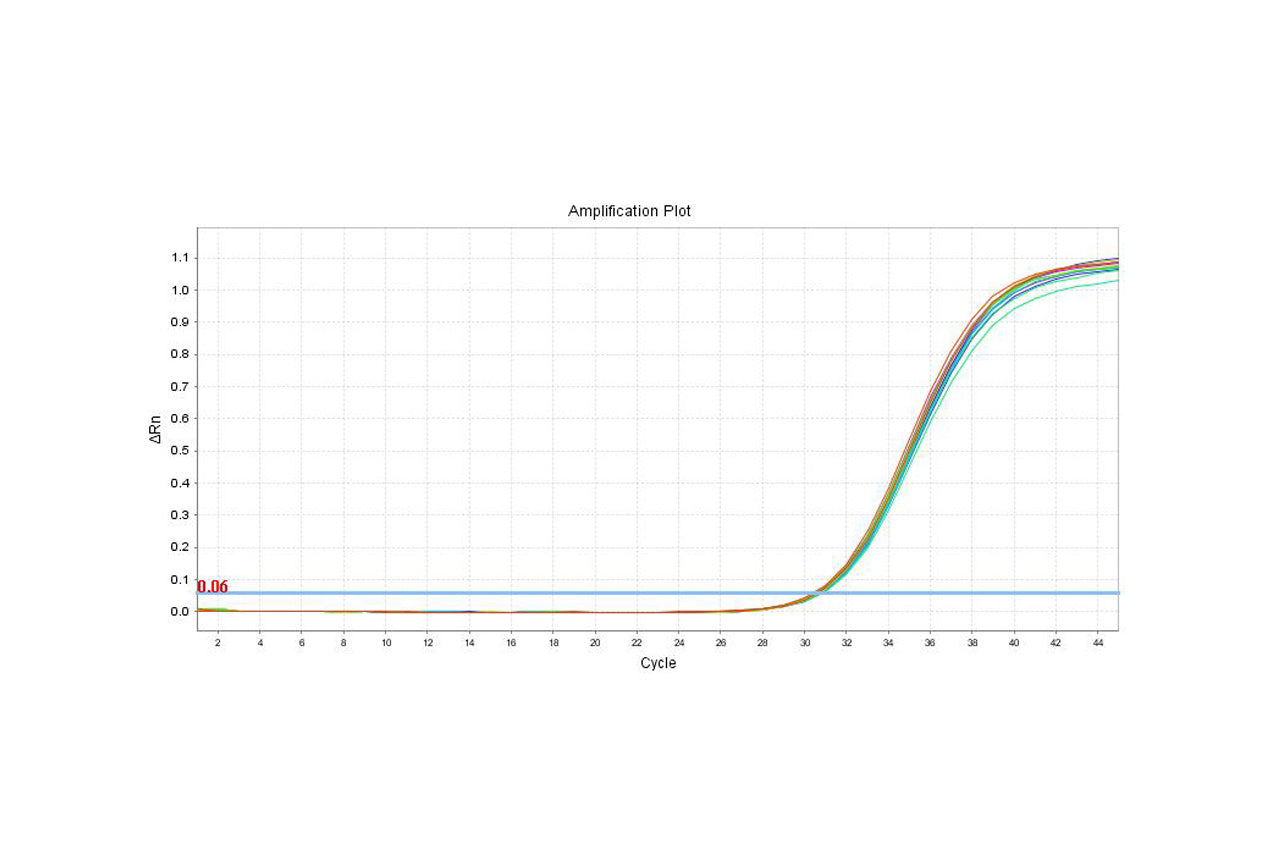
HEK293 Host Cell DNA Residue Detection Kit (3G) Validation Report
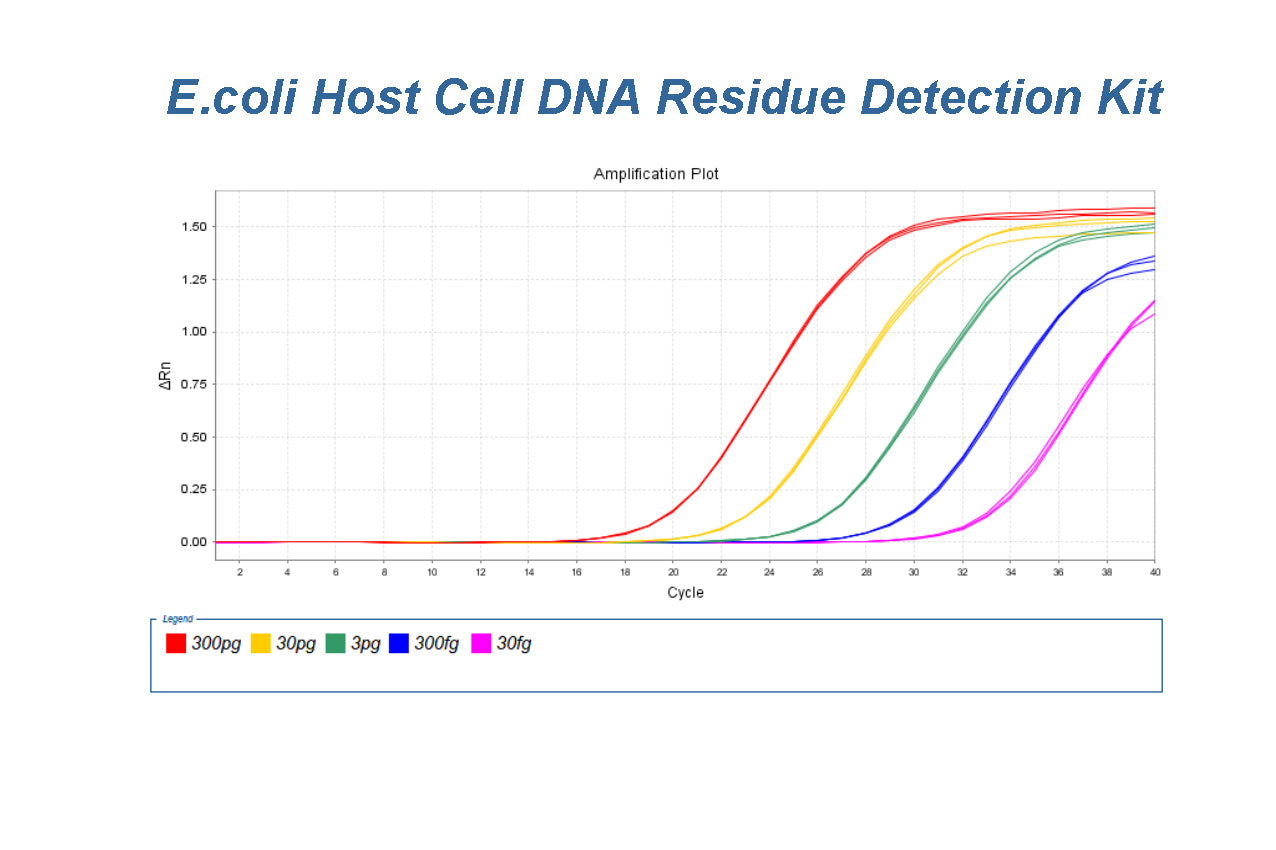
E.coli Host Cell DNA Residue Detection Kit (2G) Validation Report

Mycoplasma qPCR detection and method validation contribute to the advancement of cell and gene therapy technology development


