Description
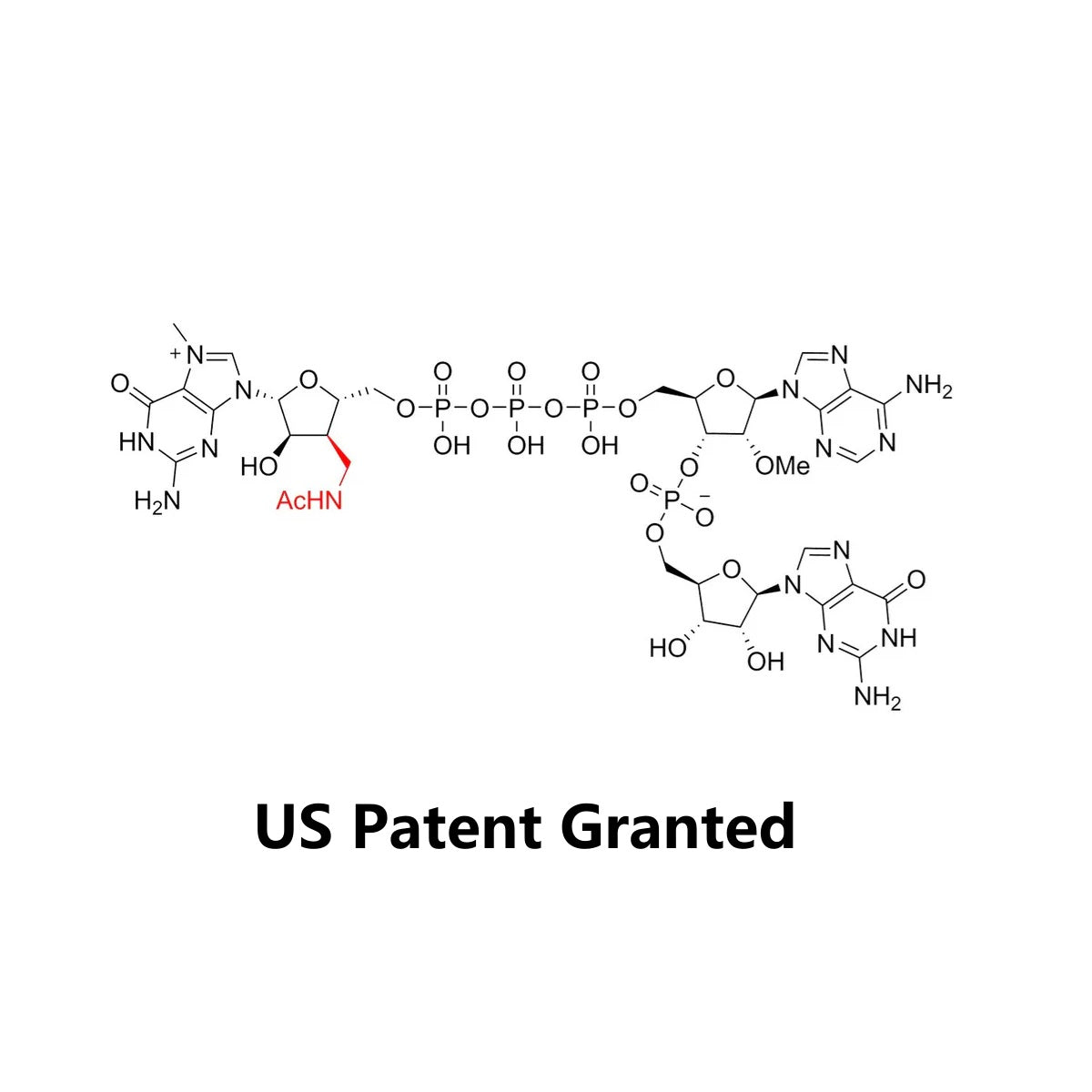
Cap AG (3'Acm) is a cap analog with the structure of m7(3'AcmG)(5')ppp(5')(2'0MeA)pG, the molecular formula of it is C35H48N16O24P4 and the molecular weight is 1200.75. The product is used for transcription with the initial sequence of 5'AG3', and the natural cap1 structure is produced by Cap1 transcription capping. Compared with Cap0 produced by traditional capping method, the cap1 structure produced by CapAG (3'Acm) enables the mRNA to have higher activity and translation efficiency in vivo.
Feature
Compared to existing products like CleanCap, this product is an 3'Acm modified mRNA cap analogue. Its double bond structure enhances molecular stability, making it resistant to recognition and hydrolysis by nucleases. With the 3'Acm, it effectively stabilizes the structure of the 5' end cap, enhancing the binding ability of mRNA with the eukaryotic initiation factor (eIF4E) and thus improving mRNA translation efficiency. Additionally, this type of cap analogue demonstrates promising data in terms of in vitro transcription yield and capping efficiency.
Components
|
Cat No. |
Name |
Size |
|
10684 |
LZCap AG (3'Acm) (100mM) |
100 μL |
|
1 mL |
Product Details
|
Molecular Formula |
C35H48N16O24P4 (Free acid) |
|
Molecular weight |
1200.75(Free acid) |
|
Concentration |
100± 3 mM |
|
Purity |
HPLC ≥95% |
Figure

Figure 1. LZCap analog showed stronger affinity to translation initiation factors eIF4E, allowing more efficient initiation of mRNA translation. The lower the Kd value, the stronger the affinity of the cap analog to the cap-binding protein eIF4E.
Figure 2. In vivo expression of LZCap AG capped mRNA is much higher than that of 3’OMe cap analog.

Figure 3. LZCap capped mRNAs are more resistant to the decapping enzyme. The higher the IC50 value, the stronger the ability of the cap analog to resist cleavage by the decapping enzyme
Figure 4. LZCap capped mRNA reduces single RNA-stimulated immune factor transcription level in THP-1 cell.
General Safety Profile
|
Result and Conclusion |
|
|
Cytotoxicity Test |
No cytotoxicity was observed with the modified nucleoside in multiple cell lines |
|
Polymerase Inhibition Study |
The modified nucleoside is neither an inhibitor nor a substrate of human RNA and DNA polymerases and is therefore not integrated into the genome |
|
Ames Test* |
No genotoxicity was observed in the Ames test |
Storage
This product could be stored at -25~-15 ℃ for two years.
Precautions
- For your safety and health, please wear personal protective equipment (PPE), such as laboratory coats and disposable gloves, when operating with this product.
- For research use only.
F&Q
1. How do you design the LZCap?
Enzymes have a relatively "specific" recognition of substrates. Therefore, when designing a new cap structure, on one hand, we need novel structural modifications for patent purposes, but on the other hand, we strive to maintain similarity with natural/known structures as much as possible. The natural structure has a ribose 3' OH, which can be modified (e.g., methylation). Based on this consideration, we chose to add a carbon at the 3' position for patent novelty, followed by an NH to mimic the hydrogen bonding of OH, and then an acetyl group to reduce the basicity of NH and enhance its hydrogen bonding capability. The activity of LzCap is better than methylated natural cap, possibly due to increased hydrogen bonding. Compared to the methyl and methoxy groups, the acetyl amino group may also increase van der Waals interactions between the substrate (cap) and the initiating factor (enzyme).
2. Is the acetyl amino group stable?
The acetyl amino group is already sufficiently stable. It is much more stable than the 7-methylated position and the phosphodiester bond, which are the least stable parts of the cap.
Publication
- Polyvalent mpox mRNA vaccines elicit robustimmune responses and confer potent protectionagainst vaccinia virus, 2024, Cell Reports 43,114269
- Preliminary Study of Efficacy and Duration of an mRNA-Based GLP-1R Agonist in Diabetic Monkeys. 2024, Diabetes, 73
Please contact us for more details about the LZCap licensing.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.