Description
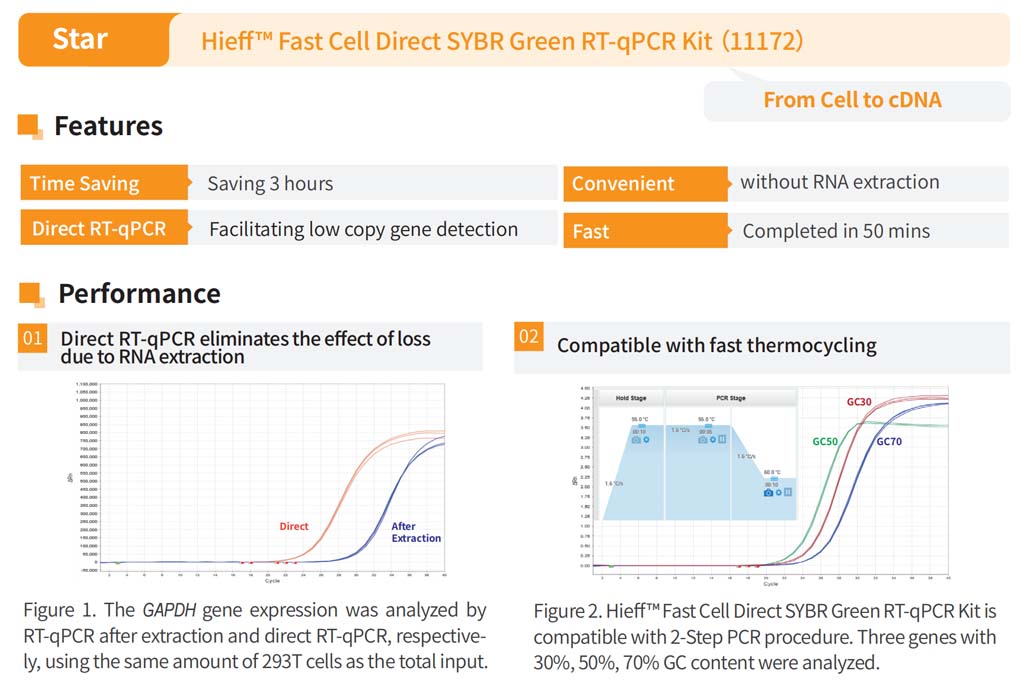
Hieff™ Fast Cell Direct RT-qPCR Kit based on SYBR Green dye is suitable for RNA extraction from all kinds of animal cells (such as cell lineage wall cells and suspension cells, primary culture cells, various stem cells, iPS cells, etc.), without the need to extract RNA, and can be used directly for qPCR expression analysis, which is short, easy to operate, and has a low error rate. It takes only 1.5 hours to complete the steps from template preparation to reverse transcription reaction and gene expression analysis.
Reverse transcription and fluorescence detection reagents are included in the kit, which can be used for gene expression analysis without the need to purchase additional reagents.
Specifications
|
Cat.No. |
11172ES40 / 11172ES60 |
|
Size |
40 T/100 T |
Components
|
Components No. |
Name |
11172ES40 |
11172ES60 |
|
11172-A |
FCD Lysis Buffer |
2 mL |
5 mL |
|
11172-B |
FCD Washing Buffer |
8 mL |
20 mL |
|
11172-C |
FCD Stop Solution |
100 μL |
250 μL |
|
11172-D |
DNase I |
80 μL |
200 μL |
|
11172-E |
4× Hifair™ FCD RT Mix |
200 μL |
500 μL |
|
11172-F |
2× Hieff™ FCD qPCR SYBR Master Mix |
2 mL |
5 mL |
|
11172-G |
RNase Free H2O |
2 mL |
5 mL |
Storage
FCD Lysis Buffer and FCD Washing Buffer were melted and stored at 4°C to prevent contamination. FCD Stop Solution、DNase I、4× Hifair™ FCD RT Mix、2× Hieff™ FCD qPCR SYBR Master Mix should be stored at -25~-15℃.
Instructions
- Preparation of cleavage products
1)1. Melt the reagent at room temperature, turn it upside down and mix it gently before use, and use it after slight centrifugation to avoid foaming*.
*Failure to mix the reagents, use of an oscillator for mixing, and failure to configure the reagents on ice can lead to a decrease in the performance of the reaction.
2)Depending on the cell type**, transfer the cells to a centrifuge tube and centrifuge at 5000 rpm for 2 min to collect the cells and aspirate the medium well. If the cells are cultured in 96-well plates, the medium can be aspirated directly.
3)Add 150 μL of FCD Washing Buffer to each well, wash the cells by blowing, centrifuge at 5000 rpm for 2 min, and aspirate the FCD Washing Buffer***.
4)Add 48 μL of FCD Lysis Buffer Solution and 2 μL of DNase Ⅰ Solution to each well, blow and mix at room temperature, then let it stand for 5 minutes, and then add 2.5 μL of FCD Stop Solution after incubation****, and then blow and mix for about 5 times to obtain the cleavage product*****.
** The basic requirement for the number of cells is 1 × 104 cells per well, and this kit can be used in the range of 1 × 103 - 1 × 106 cells. if the number of cells is larger, the amount of FCD Lysis Buffer solution and DNaseⅠsolution can be increased proportionally as appropriate.
*** Centrifugation conditions vary from cell to cell, so please centrifuge at a speed appropriate for the cells being used.
**** Add 2.5 μL of FCD Stop Solution to 50 μL of lysate, and increase the amount of FCD Stop Solution as needed.
***** For long-term storage of cell lysis product solutions, place at -20°C.
5)Melt 4 x Hifair™ FCD RT Mix at room temperature and mix with slight inversion, place on ice and configure the reaction system according to the table below:
|
Components |
Volume (μL) |
Final concentration |
|
4× Hifair™ FCD RT Mix |
5 |
1× |
|
cleavage product****** |
x |
x |
|
RNase Free H2O |
Up to 20 |
- |
*******Recommended use level is 2-5 μL, try not to exceed 45%.
- Reverse transcription
Pipette and gently mix the above prepared reaction solution and perform the reverse transcription reaction according to the procedure in the table below:
|
Temperature |
Time (min) |
|
55℃* |
15 min |
|
85℃ |
5 min |
* The recommended reverse transcription temperature is 55°C. For high GC content templates or complex templates, the reverse transcription temperature can be increased to 60°C. The reverse transcription product can be directly used for downstream RT-qPCR detection. In order to avoid the inhibition of qPCR reaction by the reverse transcription system and to get the appropriate Ct value (10-35), the product can be diluted 10-1000 times and then used. If downstream experiments are not performed for a short time, it can be placed at -20℃ for storage.
- Fluorescence quantitative PCR
1)Reaction system configuration
The following ratios are recommended for the preparation of the reaction solution (preparation on ice).
|
Components |
Volume (μL) |
Final concentration |
|
2× Hieff™ FCD qPCR SYBR Master Mix |
10 |
1× |
|
Forward Primer (10 μmol/L) |
0.4 |
0.2 μmol/L |
|
Reverse Primer (10 μmol/L) |
0.4 |
0.2 μmol/L |
|
Reverse transcription product* |
x |
- |
|
RNase Free H2O |
Up to 20 |
- |
*Do not add more than 1/10 of the RT-qPCR volume of the reverse transcription product. high concentration of template will easily lead to non-specific amplification, appropriate dilution of 5-50 times. The recommended amount of template is 4 μL, try not to exceed 6 μL. When the reaction performance is poor, the primer concentration can be adjusted in the range of 0.2-1.0 μmol/L.
2)Fluorescence quantitative PCR amplification procedure (two-step method)
|
Cycle step |
Temp. |
Time |
Cycles |
|
Initial denaturation |
95℃ |
30 sec |
1 |
|
Denaturation |
95℃ |
10 sec |
35-40 |
|
Annealing/Extension* |
60℃ |
30 sec |
|
|
Melting curve stage |
Instrument Defaults |
1 |
|
3)Rapid amplification procedure for fluorescent quantitative PCR (two-step method)
|
Cycle step |
Temp. |
Time |
Cycles |
|
Initial denaturation |
95℃ |
10 sec |
1 |
|
Denaturation |
95℃ |
5 sec |
40 |
|
Annealing/Extension* |
60℃ |
10 sec |
|
|
Melting curve stage |
Instrument Defaults |
1 |
|
* The annealing/extension temperature and final extension time can be adjusted appropriately according to the experimental requirements. The rapid program is suitable for most of the genes, and the standard program can be tried for individual complex secondary structure genes.
Notes
- This product is for research use only.
- Please operate with lab coats and disposable gloves,for your safety.
Ver.EN20230908
Documents:
11172-Hieff™ Fast Cell Direct. EN20230908.pdf
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.