Description
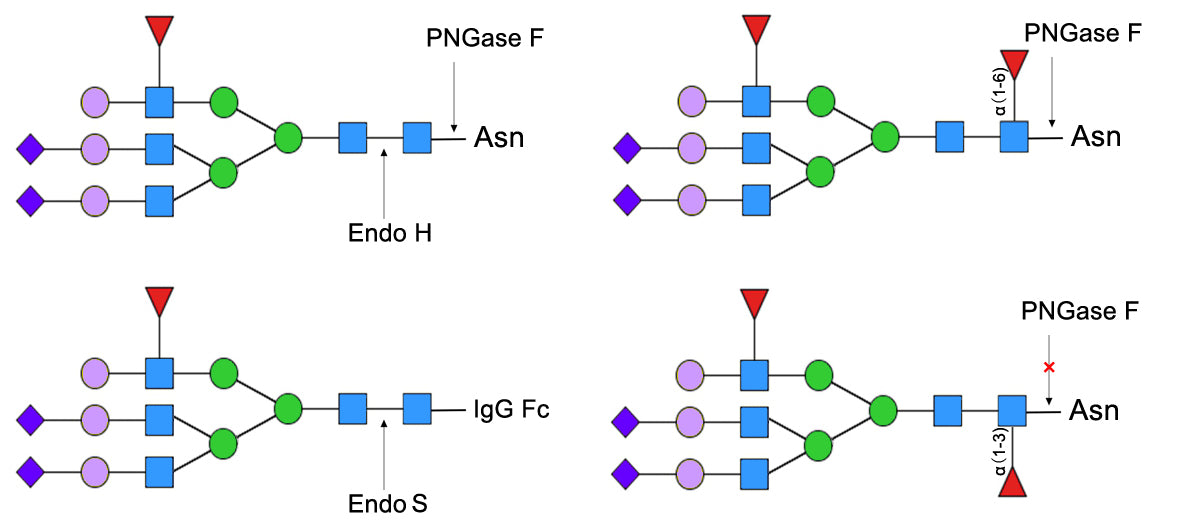
PNGase F is an amidase enzyme that is Cloned from Peace Station Eliza and mainly secreted by Neisseria meningitidis and other Gram-negative bacteria. Yeasen PNGase F is recombinantly expressed in yeast (specific activity: 100,000 U/mL), which can cleave high mannose, hybrid, and complex oligosaccharide glycoproteins linked by asparagine. The cleavage site of PNGase F is the amide bond between the inner N-acetylglucosamine (GlcNAc) and asparagine residue of glycoproteins, while converting the asparagine on the protein after enzymatic hydrolysis to aspartic acid. This product is tagged with His and is commonly used for complete deglycosylation of antibodies and related proteins.
Feature
High purity—No protease, glycosidase contamination, purity ≥95%.
High Activity— 100,000 U/mL.
Good compatibility—Can be used denaturing or no-denaturing conditions.
Application
Glycan Sequencing
Proteomics
Glycoprotein Analysis
.Recombinant Glycoprotein Expression
Specification
|
English synonym |
PNGase F; N-Glycosidase F; N-Glycosidase F |
|
Source |
Yeast recombinant expression |
|
Molecular Weight |
36 kDa |
|
Specific Activity |
100,000 U/mL |
|
Storage buffer |
20 mM Tris-HCl pH 7.5, 50 mM NaCl, 5 mM EDTA, 50% Glycerol |
|
Unit Definition |
1 unit of enzyme activity refers to the amount of enzyme required to remove more than 95% of the carbohydrates from 10 μg denatured RNase B in a 10 μL reaction system at 37°C within 1 hour. |
Components
|
Components No. |
Name |
Component Composition |
20407ES01 |
20407ES02 |
|
20407-A |
PNGase F |
PNGase F |
15000 U |
75000 U |
|
20407-B1 |
Buffer 1 (10×) |
5% SDS; 400 mM DTT |
150 μL |
750 μL |
|
20407-B2 |
Buffer 2 (10×) |
200 mM Tris, pH 7.5 |
300 μL |
1500 μL |
|
20407-B3 |
10% NP-40 |
10% NP-40 in MilliQ-H2O |
300 μL |
1500 μL |
Storage
The product can be stored at -25 to -15℃ for one year.
Figures
Documents:
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.
Inquiry
You may also like
FAQ
The product is for research purposes only and is not intended for therapeutic or diagnostic use in humans or animals. Products and content are protected by patents, trademarks, and copyrights owned by Yeasen Biotechnology. Trademark symbols indicate the country of origin, not necessarily registration in all regions.
Certain applications may require additional third-party intellectual property rights.
Yeasen is dedicated to ethical science, believing our research should address critical questions while ensuring safety and ethical standards.