How to choose drying reagents, Air Drying or Lyophilization?
As people pay more and more attention to health-related concerns, the field of in vitro diagnostics (IVD) is developing rapidly. In particular, the emergence of COVID-19 has accelerated the IVD market's expansion. At present, RT-qPCR has been widely used in the development of IVD diagnostic reagent products, but RT-qPCR liquid diagnostic reagents have high transportation costs, unstable performance, and short shelf life. Drying reagents can perfectly circumvent the shortcomings of RT-qPCR liquid diagnostic reagents. So how should drying reagents be made? How to choose the production method?
1. What are drying reagents?
2. What is air drying?
3. What is lyophilization?
4. What is the difference between lyophilization and air drying?
5. Related products and performance
1. What are drying reagents?
Drying reagents are the reagents obtained after the sample is dried and dehydrated. Drying reagents may be delivered at room temperature and do not require cold chain transportation. It can avoid repetitive freezing and thawing of reagents to affect the performance and shelf life of reagents, and greatly reduce the cost of transportation. It is not only easy for people to use, but it also lessens the demands on operators. The molecular diagnostic industry is interested in drying reagents primarily because they may be transported and stored at room temperature. There are now two widely used drying techniques: lyophilization and air drying. Air drying technology is less expensive, while lyophilization technology is the most popular, both of which have their advantages. There are two production methods of drying reagents: air drying and lyophilization. What are they and how should one choose?
2. What is air drying?
Air drying is a drying technique that can be used to prepare drying reagents. Air drying is divided into three processes: natural air drying, hot air drying, and imitation-natural air drying. In the industrial production process, to ensure the stability of product quality and output, the reagent is often dried with hot air drying. For IVD diagnostic reagents, a precision oven with a blower or vacuum function is generally used to dry the liquid at a temperature higher than room temperature (drying at 50°C for 80 minutes), The liquid is air-dried into a viscous dry material. The specific process is shown in Figure 1.
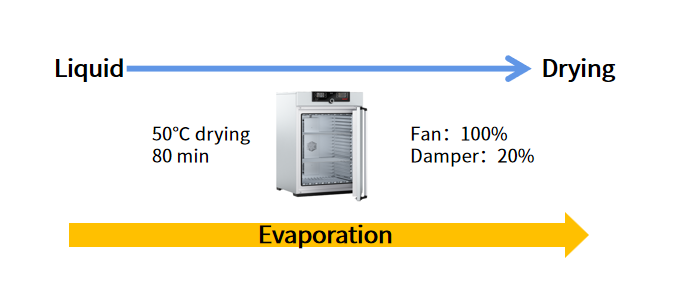
Fig 1. Hot Air Drying
The key to drying liquid molecular reagents is not only to ensure that the enzyme can be quickly hydrated after drying but also to ensure that the stability, sensitivity, and specificity of the dried reagents are not affected. Air-drying only requires a precision oven. Compared with Lyophilization, the equipment is cheaper and the energy consumption is lower, so the cost of reagents is lower. In addition, the air-drying process is simple and the drying time is shorter (within 2 hours). Air drying has both advantages and disadvantages. During the air-drying process, various components including enzymes will be lost with the evaporation of water. Not only that but the reaction solution and its components will also be harmed by the high temperature of 50 °C. Some components are also more easily oxidized, so the shelf life of air-dried reagents will be shorter. In addition, the air-drying reagent has strong viscosity and is easy to hang on the wall, and has poor rehydration capability. Lyophilization can make up for the shortcomings of air drying.
3. What is lyophilization?
Different from air drying, lyophilization is a special drying technology, whose basic principle is based on the three-phase change of water. The three phases of water are solid, liquid, and gas, and the three phases can coexist and transform each other. When the pressure is greater than 610.75 Pa, with the increase in temperature, ice melts into water, and water evaporates and turns into steam. When the pressure is less than 610.75 Pa, the ice is directly sublimated into water vapor by heating. Specifically as shown in Figure 2. Lyophilization uses the principle of phase transition of water. First, the liquid reagents are frozen to -30℃~-40℃, so that most of the moisture in the material is frozen into ice. The ice is sublimated into water vapor at a higher vacuum, providing a low-temperature heat source. The water vapor is condensed by a condenser in the vacuum system so that other material remains in the ice, thus obtaining dried products.
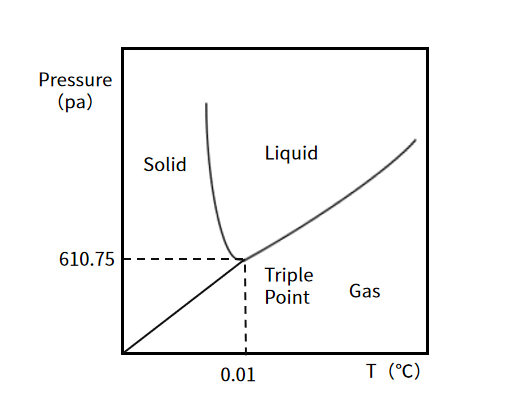
Fig 2. Phase Diagram for Water
So what are the main forms of lyophilized reagents? IVD lyophilized reagents are mainly divided into three forms: Penicillin Bottle Lyophilization, in-situ Lyophilization, and Lyophilized Microsphere. The three lyophilized formulations are shown in Figure 3. Different forms have their advantages and disadvantages. Penicillin Bottle Lyophilization is the most industrialized form. Not only has fully automatic production lines, but also it has a complete verification and risk control process. The process is the simplest and most maturest. The disadvantage is that the cost of a Penicillin bottle is high, and the filling quantity should not be too small. However, the amount of each IVD reagent is very small, so it needs to be filled for multiple servings or used multiple times at a time. If it is not used up, it needs to be stored separately, and convenience is lacking.
In-situ Lyophilization means that all components of the reagent are directly freeze-dried in the kit, which is more convenient to use. However, the distribution of cold and heat in the lyophilizer is biased, and the placement of the kits is also different, so the consistency of batches is difficult to control. In addition, the utilization rate of in-situ lyophilizers is very low, so the amortization cost is high. If a large amount of in-situ Lyophilization is performed, the lyophilized powders will be cross-contaminated.
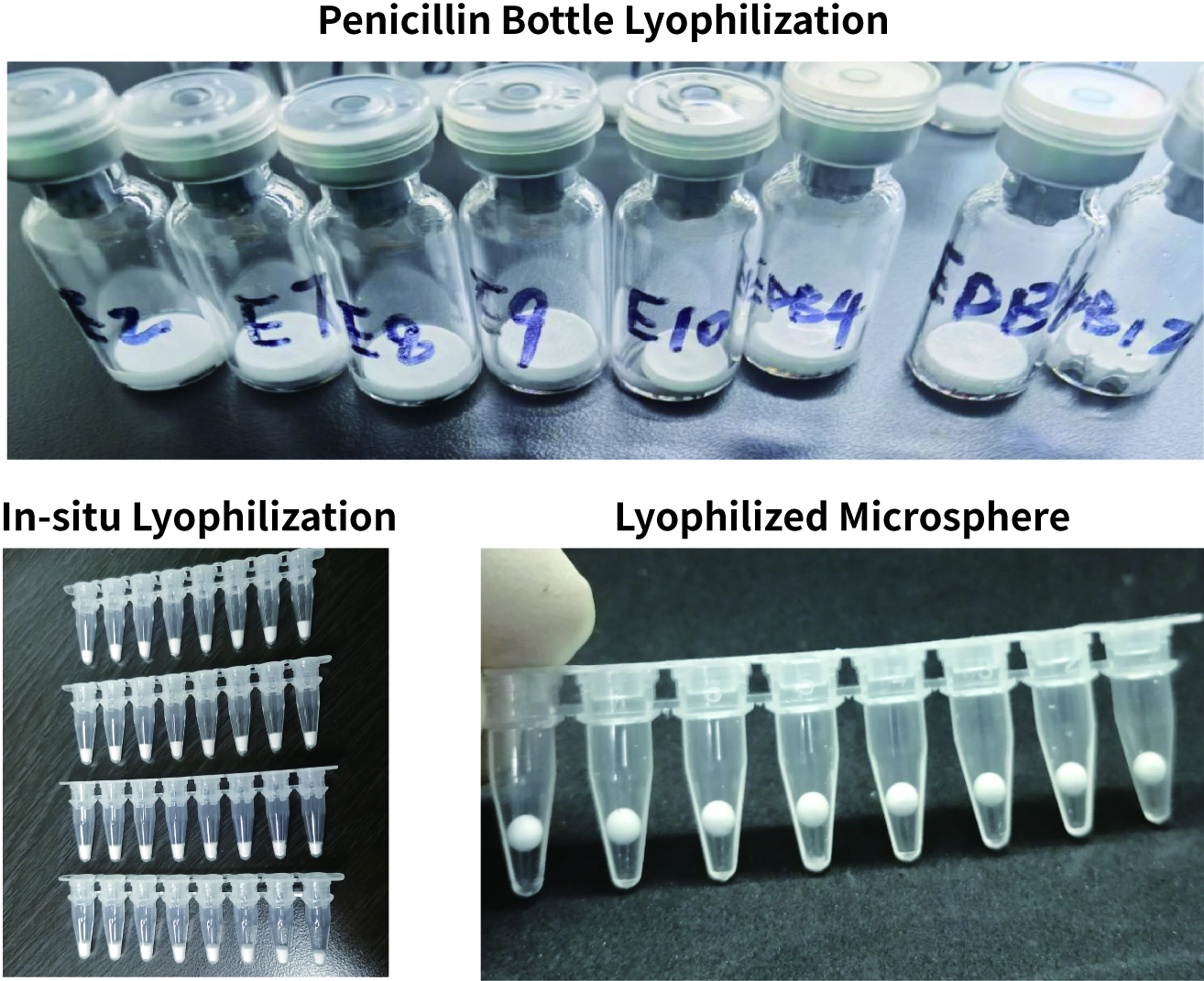
Fig 3. Form of Lyophilized Reagents
Lyophilized Microsphere can achieve accurate quantification, single person and single serving, the product is more convenient to use. It can also be treated by a special process to prevent moisture absorption, achieve room temperature storage, and solve multiple pain points at the same time. The disadvantage is that the development of Lyophilized Microsphere technology is difficult and the requirement for process control is high. Lyophilized reagents need to choose the best form according to the application scenes. Lyophilized Microsphere has unique advantages and is suitable for a variety of application scenes, which are more promising in the market.
4. What is the difference between lyophilization and air drying?
The unique process of Lyophilization determines its unique advantages. Compared with air-drying, lyophilization is superior to air-drying in many aspects. The advantages of Lyophilization are as follows:
Lyophilization is carried out at a low temperature, so the enzyme will not be denatured or lose biological activity, and biological activity is complete. Since the drying is performed in a frozen state, the volume of drying reagents is almost unchanged, and the original structure is maintained without Shrinking. When drying at a low temperature, the loss of some volatile components in the drying reagents is very small, which is suitable for reagent drying. Inorganic salts will not be precipitated on the surface of the material during drying, avoiding the surface hardening of the material. The dried material is loose and porous, dissolved quickly and completely after adding water, and restored to its original character almost immediately. Since the drying is carried out under a vacuum with very little oxygen, some easily oxidizable substances are protected. Drying can remove more than 95% to 99% of the moisture so that the dried products can be stored for a long time without deterioration.
The main differences between lyophilization and air drying are as follows:
Table 1. The Differences Between Lyophilization and Air Drying
|
Difference |
Lyophilization |
Air-Drying |
|
Process |
Freezing and Sublimation |
Hot Air and Evaporation |
|
Volume |
Hardly Shrink |
Shrink |
|
Viscosity |
Low,Not Sticky |
High, Sticky |
|
Moisture Content |
About 2% |
About 5% |
|
Rehydration Capability |
Better |
Poor |
|
Consistency |
High |
Low |
|
Stability |
24 Months |
12 Months |
|
Loss Components |
Rarely |
More |
|
Damage Enzyme |
Rarely |
More |
|
Drying Time |
About 18-24 Hours |
Less than 2 Hours |
|
Cost |
High |
Low |
|
Mass Production Difficulty |
Realizable |
Hard to Achieve |
Although lyophilized reagents cost more and take longer, the reagents produced by lyophilization are more stable and easier to use, which is exactly in line with the development direction of diagnostic reagents. Therefore, since the advent of this technology, it has become more and more popular. It has been widely used in various fields such as disease detection, virus and pathogen detection, food safety detection, animal detection, and environmental detection.
5. Related products and performance
Raw materials in IVD diagnostic reagents are the key. Non-freeze-dried materials cannot be removed directly from freeze-dried materials, and each component needs to be screened and debugged. Therefore, Yeasen Biotechnology is continuously increasing investment in this aspect and strives to provide customers with high-quality and stable lyophilized raw materials, which quickly go to market.
The related products that Yeasen can provide are shown in Table 2:
Table 2. List of Products
|
Product Name |
SKU |
Specifications |
|
11831ES60 |
100 T |
|
|
11831ES80 |
1,000 T |
|
|
11831ES92 |
10,000 T |
|
|
qPCR Lyoprotect (Inquire) |
13743ES60 |
100 T |
|
13743ES80 |
1,000 T |
|
|
13743ES92 |
10,000 T |
|
|
13743ES98 |
100,000 T |
Hifair™ Lyo Multiplex One Step RT-qPCR Kit is a glycerol-free, lyophilization-compatible (lyo-ready) liquid diagnostic reagent. This product is an ideal choice for the development of multiplex RT-qPCR that has room temperature stability and can be shipped and stored at room temperature.
Performance: The pseudovirus template was amplified by multiple RT-qPCR using liquid reagents (red) and 11831 lyophilized reagents (purple) respectively. The left graph is the FAM channel, the right is the VIC channel. The results showed that the activity of the 11831 reagents was intact after lyophilization, and it still had high-efficiency multiple reaction ability. Specifically as shown in Figure 4.
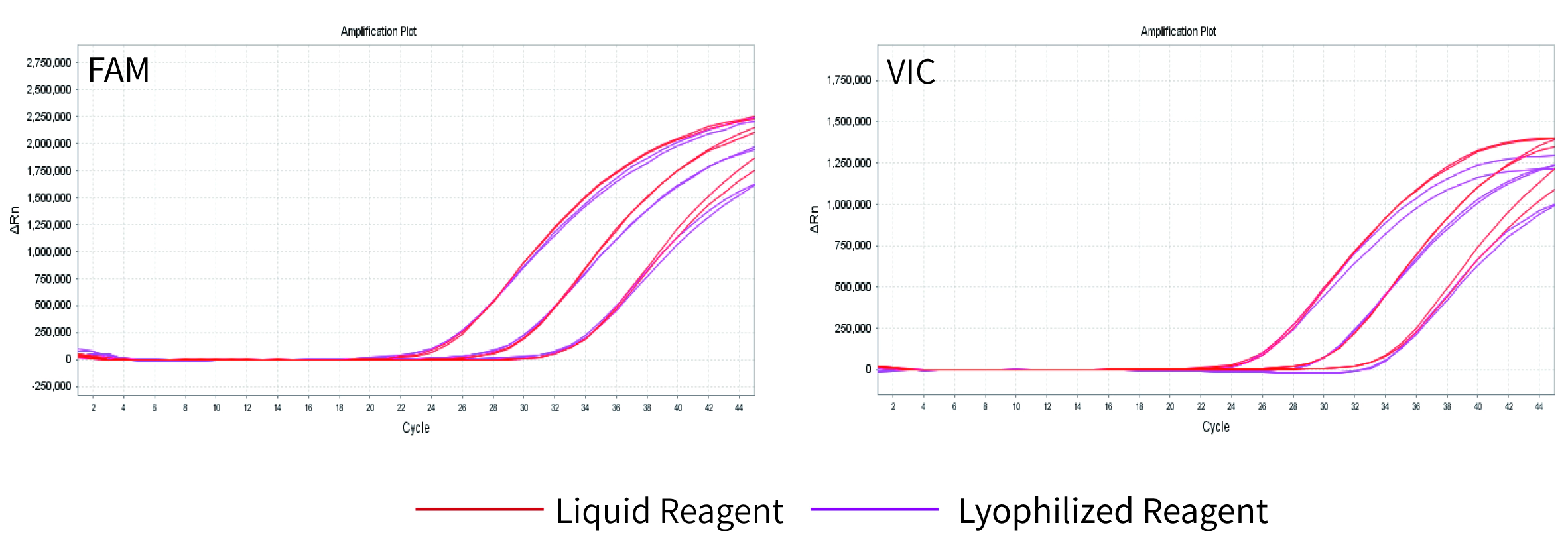
Fig 4. The Performance after Lyophilization
The 11831 lyophilized reagents was placed at 37°C (green) and -20°C (blue) for 21 days for multiple RT-qPCR amplification tests. The left graph is the FAM channel, the right is the VIC channel. The results showed that the lyophilized reagent still had a good amplification effect after being placed at 37°C for 21 days. Specifically as shown in Figure 5.
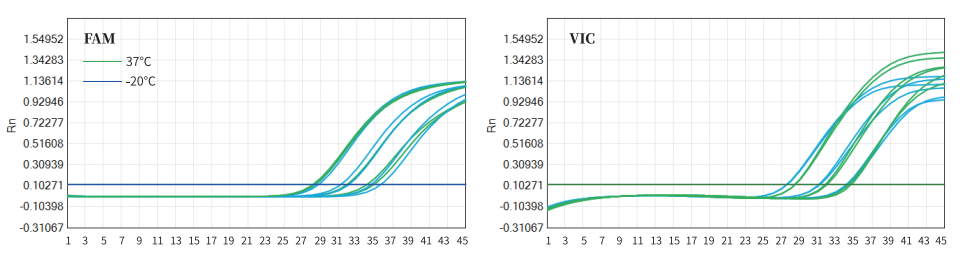
Fig 5. Thermal Stability of Lyophilized Reagent—37°C for 21 Days.
6. Product Selection Guide
|
Process |
Product name |
SKU |
|
Sample processing |
10401ES |
|
|
10325ES |
||
|
10603ES |
||
|
Reverse transcription |
11300ES |
|
|
11301ES |
||
|
PCR |
10726ES |
|
|
11893ES |
||
|
11831ES |
||
|
Hifair™ Lyo-Ready I Multiplex One Step RT-qPCR Kit (UDG plus) |
16645ES |
